Main group catalysts for reductive functionalization of CO2 into value added products
Contact person and project supervisor:
Dr. Martin Hulla, martin.hulla@natur.cuni.cz
Project Summary
The use of CO2 in chemical synthesis has the potential to decrease anthropogenic emissions of this greenhouse gas by 7-10%. However, thermodynamic and kinetic stability of CO2 necessitates the development of catalysts for its efficient use. Reductive functionalization of CO2 can be achieved with the use of main group elemental hydrides such as borohydrides and hydrosilanes. However, while useful these reducing agents fail to meet the requirements for widespread applications. Hence, the use of molecular hydrogen as the reducing agent in these reactions is highly desirable. The aim of this PhD project is the development of catalysts for reductive functionalization of CO2 using dihydrogen with applications in organic synthesis. Dihydrogen activation by main group elemental systems (B, Al, Ga, In, Si, Ge, Sn) based on the concept of frustrated Lewis pairs (FLPs) leads to elemental hydrides with structures comparable to borohydrides and hydrosilanes, which then can be used for reductive functionalization of CO2 with simultaneous regeneration of the catalyst.
The PhD project predominantly consist of synthesis of novel Lewis acids based on heavier main group elements, their applications in FLP chemistry incorporating dihydrogen and CO2 and in-situ mechanistic studies of promising catalytic systems.
Profile of the candidate
Msc. or equivalent in Chemistry, good knowledge of English
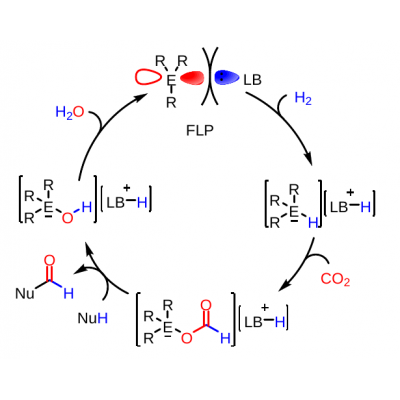
Figure 1: Hypothetical catalytic cycle for the reductive functionalization of CO2 with main group FLP catalysts
Selected publications
1) Martin Hulla, Paul J. Dyson Angew. Chem. Int. Ed., 2019, DOI: 10.1002/anie.201906942
2) Daniel J. Scott, Matthew J. Fuchter, Andrew E. Ashley Chem. Soc. Rev., 2017, 46, 5689-5700
3) Rebecca L. Melen Science, 2019, 363, 479–484
Deadline is closed